VERPACKUNGSENTWICKLUNG
Experten für Pharma Barriere Verpackung
Unsere Experten für pharmazeutische Verpackungen entwickeln, optimieren, analysieren und testen Verpackungen mit dem Ziel, die Barriereeigenschaften zu optimieren und damit die Stabilität Ihrer Produkte zu gewährleisten.
Wir fokussieren uns auf:
- Barriere und Shelf Life
- Analytische Methoden und Benchmarking
- Verpackungsvalidierung und Performance Testing
- Schadens- und Problemanalyse
Wir bieten umfassendes Know-how für pharmazeutische Verpackungen:
- Thermogeformte und kaltgeformte Blister (thermo- und coldforming)
- Stickpacks
- Pouches
- Bottles
- Injektionsfläschchen / Vials
- Spritzen / Syringes
Unser Leistungsspektrum reicht von der standardisierten Prüfung, bis hin zu unkonventionellen Analyseverfahren. Profitieren Sie von unserer Historie als ehemaliges Forschungszentrum eines Weltkonzerns und vertrauen Sie auf unser aussergewöhnliches Material-Know-how, unsere grosse Expertise und unsere erstklassige Laborinfrastruktur.


Verpackungsentwicklung
Fortschritt durch Materialkompetenz
Die Ansprüche an pharmazeutische Verpackungen sind vielfältig und hoch. Die vorwiegend aus Kunststoff sowie Aluminium hergestellten Verpackungen müssen hohe funktionale Anforderungen beispielsweise bezüglich Barriere, Siegelbarkeit oder Umformbarkeit erfüllen. Ausserdem gelten immer höhere Anforderungen bezüglich Recyclierbarkeit und der Verzicht auf Halogene wie z.B. Chlor oder Fluor.
Die hohen Anforderungen stehen oft im Widerspruch zur Material- und Kostenreduktion. Folglich sollen die Werkstoffe bezüglich ihrer physikalischen und mechanischen Eigenschaften möglichst ausgeschöpft werden. Dies setzt ein fundiertes und umfassendes Materialverständnis voraus.
Suisse Technology Partners AG ist als unabhängiger Dienstleister ein langjähriger Partner der Pharmabranche und unterstützt seine Kunden in der Verpackungsentwicklung, der Produkt- und Prozessoptimierung oder bei Schadenfällen (Schadensanalyse).
Barriere und Shelf-Life
Um die Funktionalität und Haltbarkeit von pharmazeutischen Produkten zu garantieren, muss der Inhalt vor äusseren Einflüssen wie Feuchtigkeit oder Sauerstoff und Temperatureinwirkungen geschützt werden.
Die Barriere von thermogeformten wie auch von kaltgeformten Blistern kann mittels numerischer Simulation sowie spezifisch entwickelten analytischen Berechnungsmethoden ermittelt werden.
BARRIEREBERECHNUNG VON THERMOGEFORMTEN BLISTERN

Analytische Barriereberechnung (WVTR, OXTR) auf Basis von Dickenmessungen an bestehenden Blistern
Numerische Barriereberechnung (WVTR, OXTR) mittels FEM (Finite Elemente Methode).
Bei thermogeformten Blistern hat der Umformprozess eine direkte Auswirkung auf die Barriereeigenschaften der Verpackung und folglich auf die Haltbarkeit des Produkts. Durch den Einsatz von numerischen Simulationsmethoden können Vorhersagen gemacht werden, ohne in teure Formwerkzeuge investieren zu müssen.
Die dafür benötigten Materialeigenschaften werden in speziell entwickelten Testverfahren in den eigenen Labors ermittelt.
BARRIEREBERECHNUNG VON KALT GEFORMTEN BLISTERN (QUERDIFFUSION)
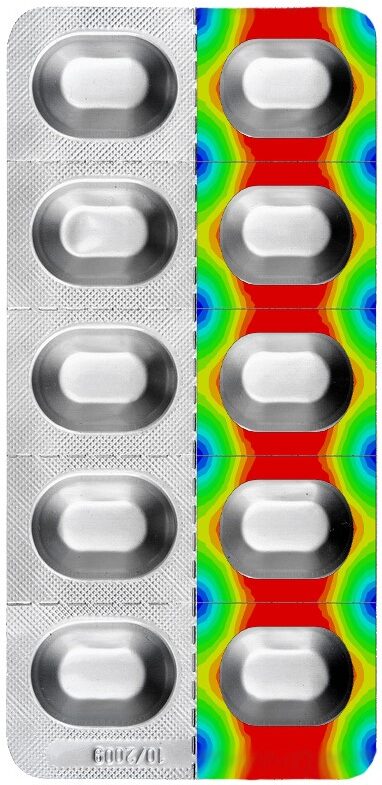
Obwohl Aluminium als 100-prozentige Barriere gegen Wasserdampf und Sauerstoff betrachtet wird, kann bei einem Aluminiumblister Feuchtigkeit durch die Siegelschicht eindringen und das Füllgut beschädigen bzw. dessen Lebensdauer reduzieren. Diese sogenannte Querdiffusion (cross diffusion) ist messtechnisch an einem Blister nur mit relativ grossem Aufwand zu bestimmen. Suisse Technology Partners hat jedoch ein Verfahren entwickelt, um die Querdiffusion durch Simulation am Computer vorherzusagen.
Die dafür benötigten Materialeigenschaften werden in einem speziell entwickelten Testverfahren ermittelt.
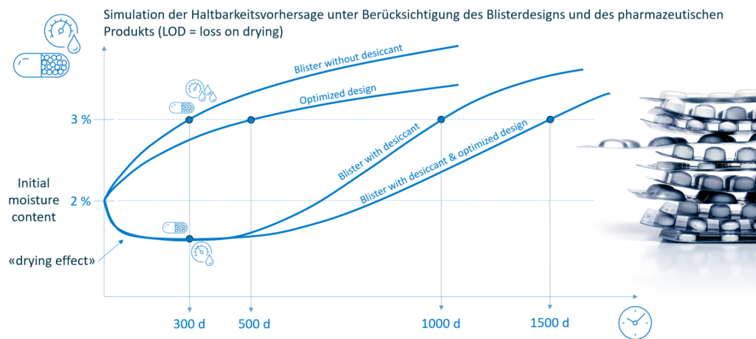
Zusätzlich kann unter Einbezug der Sorptionseigenschaften des Füllguts dessen Feuchteaufnahme (LOD: loss on drying) bzw. die Haltbarkeit berechnet werden.
SHELF-LIFE VORHERSAGE
Basierend auf der Barriere der Verpackung und unter Berücksichtigung der physikalischen Eigenschaften des Produkts ist direkt die Vorhersage dessen Haltbarkeit möglich. Damit lassen sich aufwändige Stabilitätstests abkürzen oder gar vollständig ersetzen.
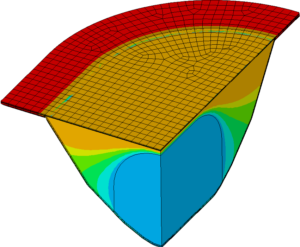
THERMAL IMPACT ANALYSE
Mittels numerischer Simulation wird der thermische Einfluss des Siegelprozesses auf das Füllgut berechnet. Damit lässt sich eine mögliche Degeneration des Produkts durch Wärmeeintrag im normalen Prozess aber insbesondere auch bei unvorhergesehenen Abläufen wie Produktionsunterbrüchen bestimmen.
- Vorhersage der zeitabhängigen Temperaturverteilung im Blister
- Untersuchung allgemeiner Fragen der Wärmeübertragung
Analytische Methoden
Für die Analyse einer Verpackung im Entwicklungsprozess, im Schadensfall oder im Rahmen der Qualitätssicherung steht eine Vielzahl von Methoden zur Verfügung.
Neben optischen Prüfungen mittels Lichtmikroskopie stehen auch die Rasterelektronenmikroskopie (REM/EDX) und viele weitere materialographische, physikalische und chemische Untersuchungsmöglichkeiten zur Verfügung.
Die Vielfalt der Analysemethoden, gepaart mit der langjährigen Erfahrung der Experten von Suisse Technology Partners, ermöglichen eine erfolgreiche Unterstützung bei komplexen Fragestellungen.
BENCHMARKING VON VERPACKUNGSFOLIEN UND VERPACKUNGEN
Als unabhängiges Dienstleistungsunternehmen charakterisieren und vergleichen wir Aufbau, Materialzusammensetzung und Performance etc. von Verpackungsfolien (Halbzeugen) oder Verpackungen mit den geeigneten Analysemethoden.
Multiylayer Analyse eines thermogeformten Barriereblisters
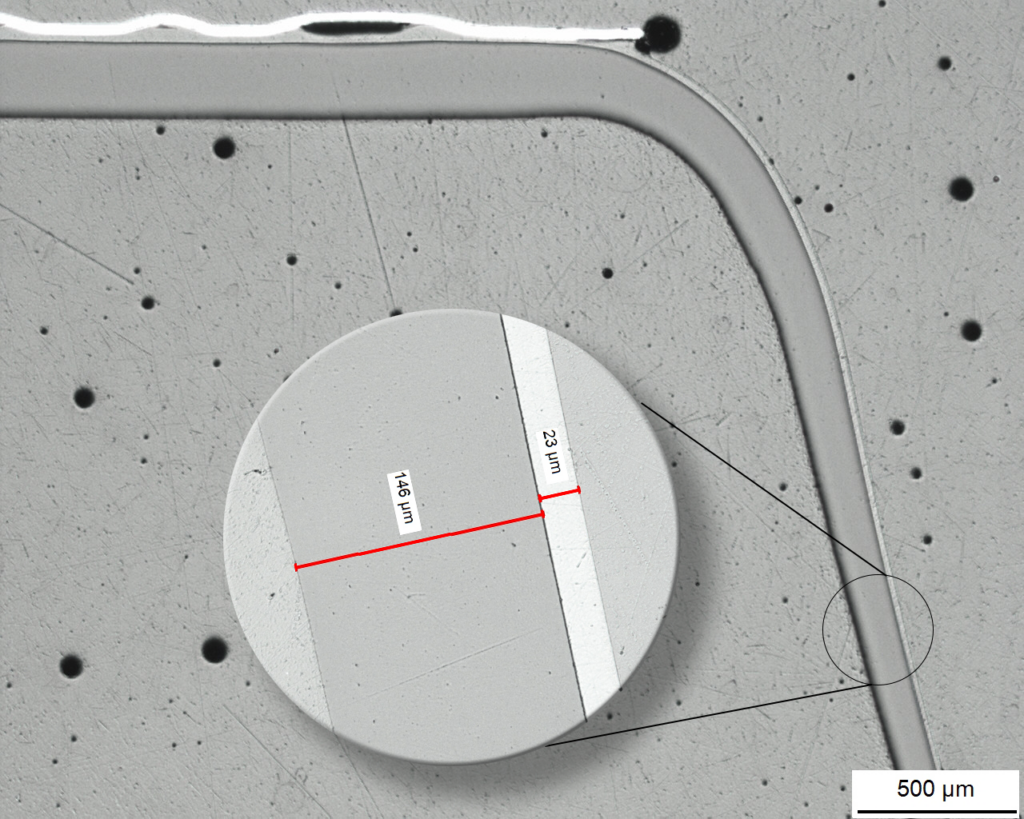
Schliffbild einer Kavität
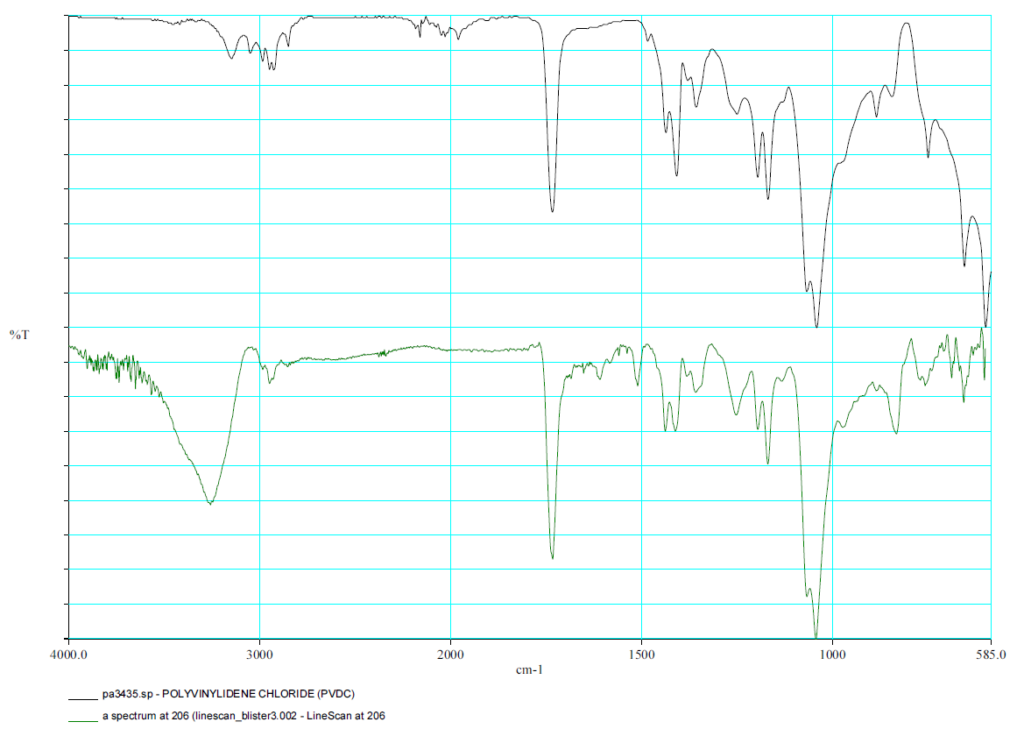
FTIR Spektrum PVDC
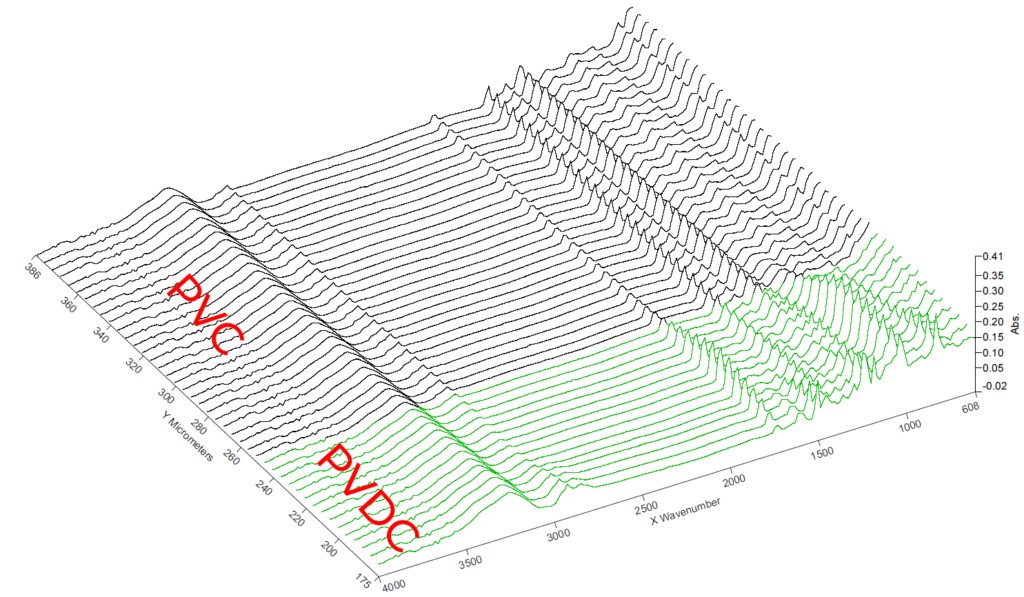
FTIR - Linescan
Forming Limit Analyse
Mittels optischer Dehnungsmessung werden Umformzustände und Oberflächendehnungen von Alu-Alu (coldform) Blistern bewertet.
Das Forming Limit Diagramm (FLD) zeigt auf Basis der Dehnungsmessung und der Umformbarkeit des Materials (Forming limit curve, FLC) die Tendenz zur Rissbildung. Diese kann als Mass für die Prozessstabilität verwendet werden.
VERPACKUNGSVALIDIERUNG UND PERFORMANCE TESTING
Wir unterstützen Sie im Rahmen der Validierung von Verpackungen und Verpackungsprozessen. In unseren sehr umfangreichen Labors führen wir sowohl standardisierte als auch kundenspezifische Prüfungen durch:
- Dichtigkeitstests (z.B. Dye leak test)
- Siegelnahtintegrität, Siegelnahtdichtigkeit
- Siegelnahtfestigkeit (ASTM F88, akkreditierte Methode)
- Analytische Untersuchung der Siegelnaht mittels 3D Computertomographie CT, Mikroskopie oder REM mit EDX
- Dickenmessung an Folien und Verpackungen
- Beschleunigte Alterung, Klima– und UV-Tests
- Mechanische und physikalische Prüfungen
- Schadens- und Problemanalysen
- Polymertechnische und chemische Untersuchungen (Infrarotspektroskopie FTIR mit FTIR-Microskop, TGA, DSC, DMA, TMA, Rheometer, Kalorimeter, Coulometer, Wassergehalt etc.)
- Messung der Querdiffusion (Bestimmung der Permeationseigenschaften)
- WVTR Messung nach USP 671
- Kundenspezifische Tests

SCHADENS- UND PROBLEMANALYSE
Die Schadensanalyse ist ein systematisches Verfahren zur Ermittlung der Ursache des Versagens. Erfahren Sie mehr darüber unter Schadensanalysen.
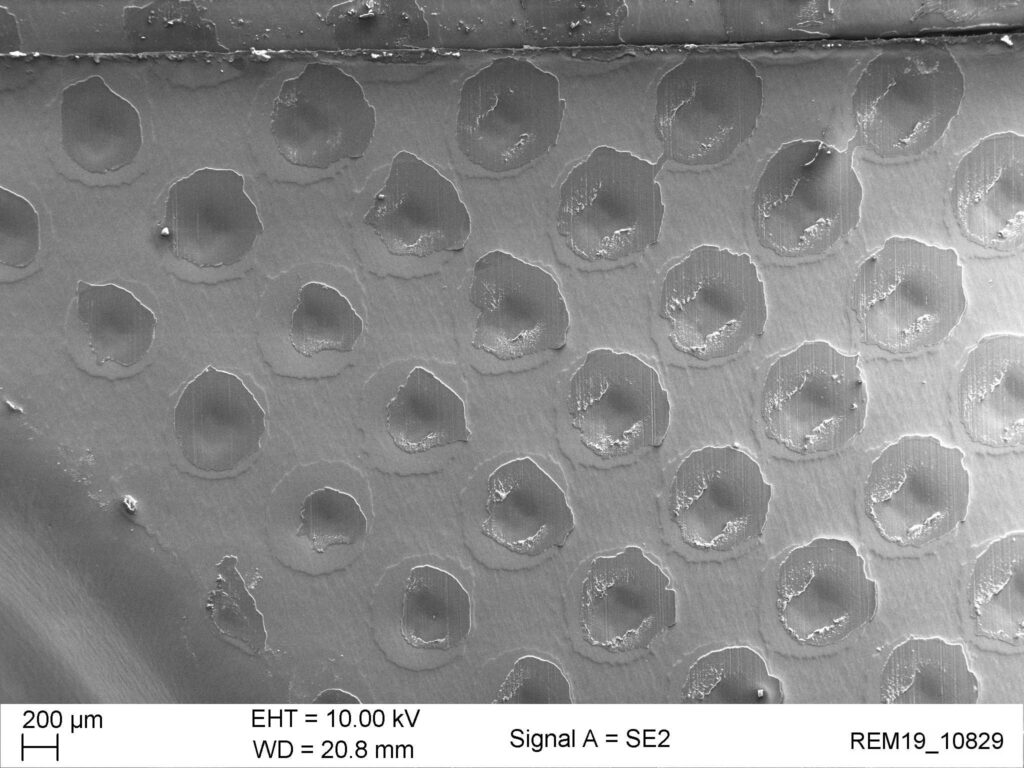
Beispiel der Röntgenprüfung der Siegelnaht eines Hochbarriereblisters mittels Kontrastmittel. Undichte Stellen sind auch über grössere Flächen sehr gut erkennbar. Die REM Bilder zeigen die Ausbildung und Qualität der Siegelnaht mit sehr hoher Auflösung.
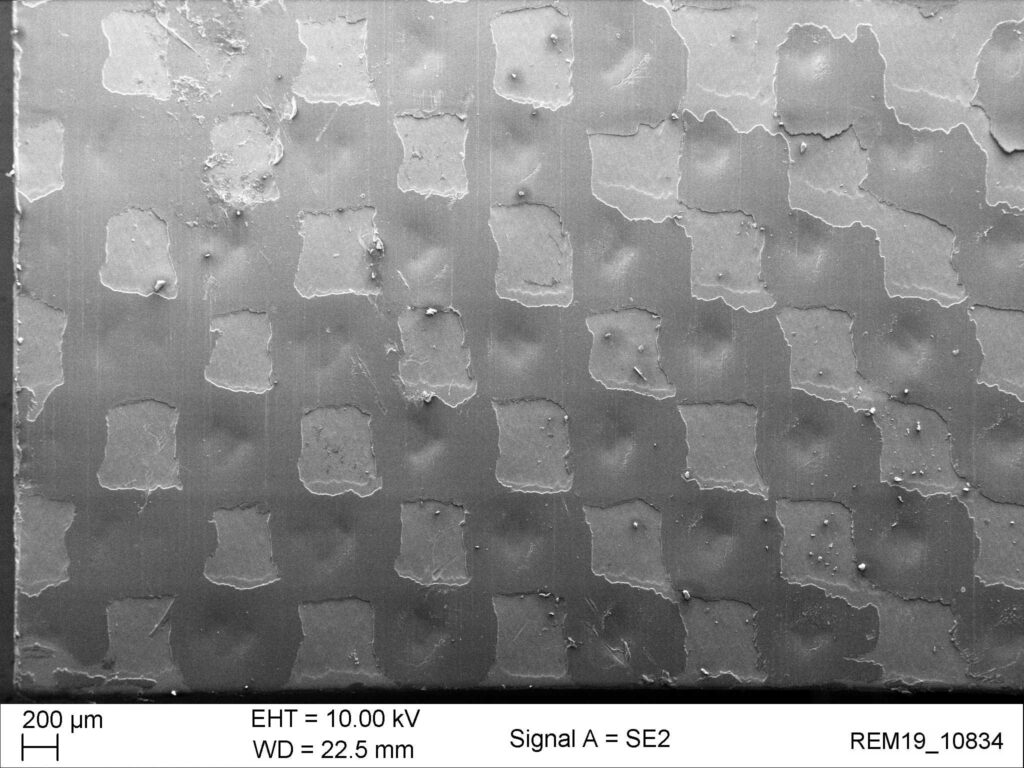
intakte Siegelung
Bei einer intakten Siegelnaht verbinden sich die Klebepunkte so, dass eine geschlossenporige Siegelfläche entsteht.
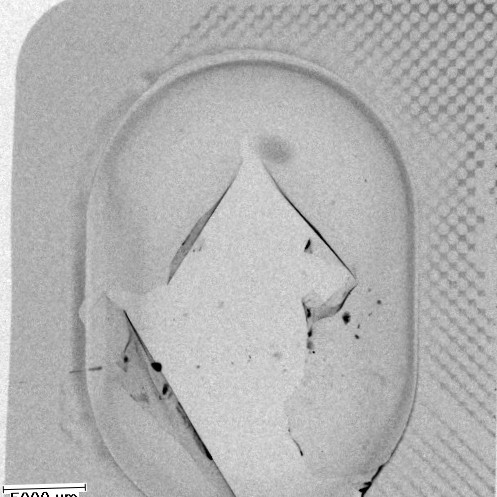
2D - CT Aufnahme mit Kontrastmittel