WE ARE COMMITTED TO QUALITY
Accredited according to:
- GMP-certified by Swissmedic
- FDA established (identifier of the facility 3008262422)
- ISO/IEC 17025 accredited (STS 0023) by the Swiss Accreditation Service
ISO/IEC 17025 accredited (STS 0023) by the Swiss Accreditation Service
The Swiss Accreditation Service SAS examines and accredits conformity assessment bodies (CABs) on the basis of the relevant international standards. Based on the Accreditation and Designation Ordinance of June 17, 1996 and on the advice of the Swiss Federal Accreditation Commission, the Swiss Accreditation Service (SAS) grants Suisse Technology Partners AG accreditation as a testing laboratory for chemical, physical and mechanical testing of metallic materials, environmental samples and elements in organic materials and salts.

GMP-certified by Swissmedic
Suisse TP is duly authorized by Swissmedic, the Swiss Agency for Therapeutic Products, to manufacture medicinal products. This authorization allows Suisse TP to carry out pharmaceutical analyses and manufacture active pharmaceutical ingredients (API) for use in clinical trials within the framework of good manufacturing practice (GMP). The certificate is based on inspections carried out in accordance with the requirements of good manufacturing practice and quality control of the Pharmaceutical Inspection Convention/Cooperation Scheme (PIC/S) and the guidelines of the European Commission.

FDA established
The US Food and Drug Administration (FDA) has successfully inspected Suisse Technology Partners AG. Accordingly, Suisse Technology Partners AG has been recognized and registered by the FDA as a testing laboratory for the analysis of pharmaceutical products. The registration number for Suisse Technology Partners AG is FEI 300 826 2422.
In addition, Suisse Technology Partners AG has successfully passed the initial self-identification according to the Generic Drug User Fee Amendments (GDUFA) and has been accepted by the FDA. Suisse Technology Partners AG undertakes to maintain this status and to confirm the required identification information to the FDA on an annual basis.

Handling controlled substances - SwissMedic
Suisse Technology Partners has a federal license to handle controlled substances in accordance with Art. 2 let. h BetmKV.
This ordinance regulates the licensing and control of narcotics, psychotropic substances, precursors and auxiliary chemicals in accordance with Art. 2 BetmG and of raw materials and products with a narcotic-like effect in accordance with Art. 7 BetmG.

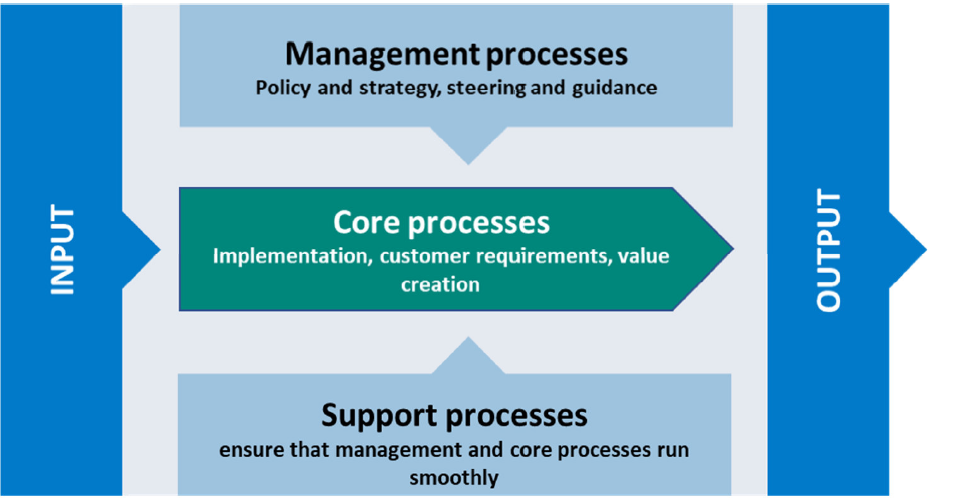
CENTRALIZED ADMINISTRATION - EFFICIENCY AND EFFECTIVENESS
Our certified, customer and process-oriented Integrated Management System (IMS) combines relevant management areas such as quality management, risk management, data protection management, knowledge and idea management and EHS (Environment, Health, Safety) management in a uniform management concept.
INTERNAL AND EXTERNAL MONITORING
Rigorously conducted internal and external audit procedures are a must. Internal audits investigate and resolve issues related to our procedures and processes, and our external audit not only helps to ensure that the organization complies with applicable laws, regulations and standards, but also provides independent testing and analysis of our internal controls.Suisse TP has been successfully audited and qualified by many clients.




