Water determination by Karl Fischer titration
Titration for water determination according to Karl Fischer. This method can be used to determine both traces of water and the percentage water content in almost all samples, whether liquid, solid or gaseous. Coulometric KF titration is used to detect water in the trace range. Volumetric KF titration, on the other hand, is used to determine the water content in %. Karl Fischer is the
In KF titration, a Karl Fischer reagent solution, which undergoes a specific chemical reaction with water, is used to determine the water content. It is a frequently used method in moisture analysis, especially when it comes to measuring very low water contents. In contrast to conventional titration, Karl Fischer titration uses a specific reagent that reacts selectively with water.
investigation method
- Water content determination in the % and trace range using volumetric or coulometric Karl Fischer titration.
- Use and evaluation via the tiamo software.
Device type and equipment
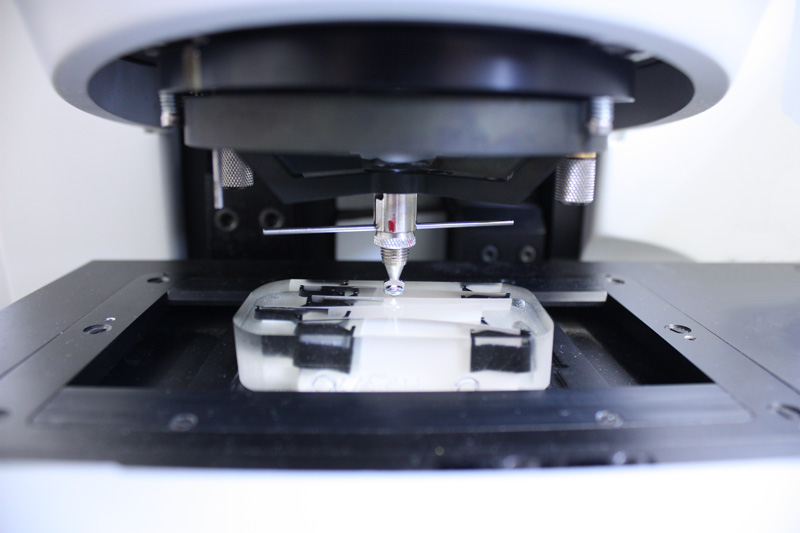
- Metrohm 852 Titrando with 874 furnace
- Metrohm 836 Titrando
functional principle
- In the coulometric Karl Fischer titration, iodine is generated electrochemically during the titration and reduced in a further reaction with water consumption. This reaction can only take place as long as water is present in a sample (= end point of the titration).
- For higher water contents, the volumetric Karl Fischer titration is used, in which a solution containing iodine is added to the sample solution using a burette.
Typical applications
Water determination in liquids, solids and gases (e.g. pharmaceutical products, plastics, papers, films and foils, lacquers, metal oxides and salts, foodstuffs, inorganic salts (crystal water)) coulometric in the trace range (1ppm -5%) and volumetric at higher water contents (in the % range).
Norms and standards
Various chapters and monographs from EP and USP; in the field of chemistry and materials testing, e.g. BS 7912, DIN 51777, DIN EN 14214, ISO 12937
Requirements for sample
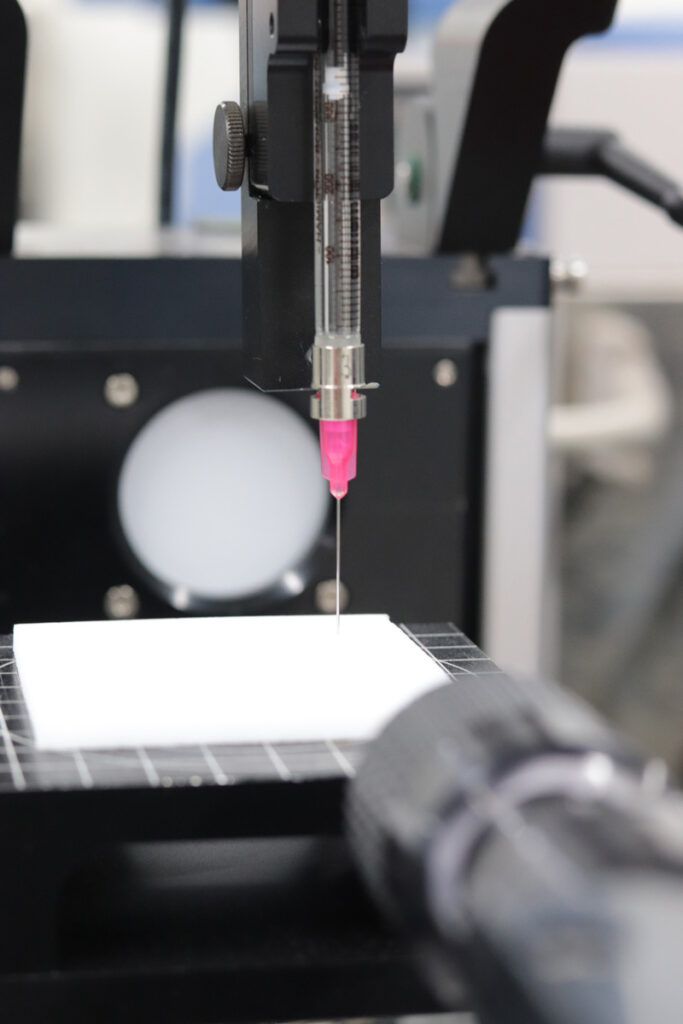
from 10 mg, depending on the water content of the sample
Karl Fischer titration methods
The Karl Fischer method for determining water content is one of the most frequently used titration methods.
Today, there are two different methods for determining water content according to Karl Fischer: volumetry and coulometry.
The appropriate titration method is selected based on the estimated water content in the sample:

Volumetric Karl Fischer titration
During the titration, iodine is added by means of a burette. Suitable for samples in which water is a significant component: 100 ppm – 100 %

Coulometric Karl Fischer titration
Iodine is generated electrochemically during the titration. Suitable for samples containing trace amounts of water: 1 ppm – 5 %
Example applications for water content determination
- Pharmaceutical industry: Determining the water content in drugs and pharmaceutical products, as the water content can affect the stability and quality of the products.
- Food industry: Analyzing the water content in food products such as grains, dried fruits, and dairy products to assess shelf life and quality.
- Chemical industry: Monitoring the water content in chemical reagents, solvents, and products to ensure their purity and usability.
- Environmental analysis: Determining the water content in soil samples, wastewater samples or environmental samples to assess moisture levels and potential pollutant loads.
- Electronic industry: Measuring the water content in electronic components and materials, as moisture can affect electrical performance and reliability.
These examples illustrate the wide applicability of Karl Fischer titration for the accurate determination of water content in various industries.
Methods that might also interest you
These also include, among others, chromatographic, spectrometric, thermo/mechanical and other methods:

Contact Me
Patrik Bachmann
Laboratory Manager Chemical Analysis