HPLC laboratory
Our HPLC laboratory (and also UPLC laboratory) is part of our modern infrastructure, in which an independent CRO Team offers solutions for pharmaceutical and chemical companies.
Our laboratory is staffed by a highly experienced team of chemists, engineers and laboratory technicians who have many years of R&D, analytical and project management experience in global pharmaceutical companies. More than half of the team members have a doctorate in science.
HPLC & UPLC is used in our laboratory primarily for the content and purity determination of pharmaceutical products.
What is HPLC? - Basic principles
HPLC and UPLC are both liquid chromatographic separation methods (LC = Liquid Chromatography). This means that a liquid (mobile phase or eluent) is pumped through a separation column (stationary phase). If a dissolved mixture of substances is injected into such a chromatographic system, it can be separated into its individual components by interactions with the stationary phase.
After passing through the column, the separated components or individual substances reach a detector that outputs a signal proportional to the component quantity, graphically as a so-called chromatogram. Ideally, each peak of a chromatogram represents a component of the substance mixture.
Both HPLC and UPLC can be used to identify and quantify substances or separated components of a substance mixture. In the case of GPC (Gel Permeation Chromatography) or SEC (Size Exclusion Chromatography), a special sub-type of liquid Chromatography, it is also possible to determine the molecular weight distribution by separating a substance mixture according to the different sizes of the individual components.
The basic instrumental configuration of HPLC and UPLC is the same in principle. Both variants of liquid chromatography consist of the following instruments:
- eluent reservoir
- pump (isocratic/gradient)
- sample application (injector)
- column
- detector
- data recording and control software
What is the difference between HPLC and UPLC?
When the mobile phase is pumped through a stationary phase, relatively high pressures are generated in the chromatographic measuring system. This is why HPLC technology was also known as high pressure liquid chromatography in its early days.
HPLC (High Performance Liquid Chromatography) typically works with column pressures of up to 400 bar (approximately 6,000 psi), while UPLC (Ultra Performance Liquid Chromatography) can work at higher pressures of around 1000 bar (15,000 psi).
The higher pressures in UPLC compared to HPLC are due to the use of smaller particles of column material. This development towards ever smaller particulate packing materials for the separation columns initiated the change from HPLC to UPLC. While particle sizes of three to five micrometers are used in classic HPLC, UPLC columns are packed with particles that are often only 1.5 µm in size.
The miniaturization of particles in UPLC has increased the performance of HPLC technology many times over, as it has been possible to significantly reduce analysis times and increase separation efficiency, thus enabling higher sample throughputs with smaller sample quantities and less eluent consumption. In addition, a reduction in the detection limits of substances can be achieved with concentration-dependent detectors.
However, miniaturization also led to higher instrumental demands on a UPLC system (including pressure-stable materials and construction methods such as high-pressure capillary connections, minimized volumes such as dead volumes, detector flow cells).
What separation mechanisms are there?
In HPLC and UPLC, the separation of a mixture of substances occurs on the separation column, whereby the separation is based on the different distribution of the sample substances between the stationary phase (separation column) and the mobile phase (eluent).
On the one hand, these interactions are interactive interactions of the sample with the stationary phase. This is referred to as adsorption chromatography. The adsorptive interactions are based, among other things, on the polar or non-polar properties of the sample and column material (van der Waals interactions) or on electrical properties.
Typical examples of adsorption chromatography are:
normal phase chromatography (NP)
NP is characterized by the fact that the stationary phases have polar surface properties and eluents with a non-polar character are used. Under these conditions, polar functional groups of samples can interact optimally with the column material and be separated from each other.
Hydrophilic interaction liquid chromatography (HILIC)
In HILIC, the analytes are very polar compounds, which is why HILIC can also be seen as an extension of NP. HILIC stationary phases are highly polar and are used with aqueous mobile phases. Gradient methods start with a fairly high organic content and end with a higher aqueous content.
reverse phase chromatography (RP)
As the name suggests, the stationary phases are non-polar in contrast to NP, and the mobile phases are more polar in character. Gradient methods start with a fairly high aqueous content and end with a higher organic content. RP is currently the most commonly used separation technique in liquid chromatography.
Ion (exchange) chromatography (IEX)
IEX is used for electrically charged samples and is characterized by the interaction with oppositely charged surface particles of the stationary phase. Mobile phases are buffer solutions whose elution power can be influenced, for example, by their composition and pH value.
Chiral chromatography (enantiomeric separation, CLC)
The problem with the separation of enantiomers is that they have almost identical physico-chemical properties and therefore cannot be separated using the classic adsorptive NP or RP methodologies. In CLC, therefore, use is made of the property of enantiomeric compounds of having chiral centers. When such chiral centers react with a counterpart on the stationary phase or a suitable chiral eluent additive to form “diasteromers”, these can be separated chromatographically.
Hydrophobe Interaktionschromatographie (HIC)
HIC means that salting out of a relatively polar sample forces hydrophobic interactions between the sample and the stationary phase. This method is used primarily in protein chromatography, since polar protein molecules originally lose their hydration shell at high salt concentrations and can interact with their nonpolar molecular portions on a moderately nonpolar stationary phase. Gradient methods in HIC start with a fairly high salt content/ion
Affinity Chromatography (AC)
The AC method is also used to separate biomolecules. The interaction between the sample and the stationary phase takes place by exploiting very specific properties of a biomolecule and selecting the stationary phase specifically for this specific property (e.g. biomolecule <-> suitable antibody on the stationary phase; enzyme <-> suitable inhibitor,
In addition to interactive chromatography, there is also non-interactive chromatography. The aim here is to avoid interactive interactions of the sample with the stationary phase. A typical example of this type of chromatography is
Gel permeation chromatography (GPC/SEC)
GPC/SEC separates a mixture of substances according to the different sizes of the individual sample components. The stationary phase in GPC/SEC herefore does not work according to the adsorption principle, but optimally only through a sieving effect of the pores of the column material. Small molecules in a mixture of substances will be able to penetrate the pores of the GPC column material completely and will only be eluted later, while medium-sized or large molecules will only partially penetrate the pores and thus leave the column earlier. Depending on the sample properties, GPC/SEC can be carried out with aqueous or organic eluents.
What HPLC detectors are there?
After leaving the stationary phase, the separated sample components must be detected in order to be able to evaluate them qualitatively and quantitatively.
In HPLC and UPLC, a variety of detectors are available for this purpose, which can be divided into selective and non-selective or universal detectors.
A detector whose electronic signal is proportional to a certain property of a sample and/or the mobile phase, for example, belongs to the non-selective/universal detectors. It responds to practically every compound in the sample that it is capable of detecting.
Non-selective Typical examples of non-selective/universal detectors include:
REFRACTIVE INDEX DETECTORS RI Detector
Refractive index detectors register all substances that exhibit a change in the refractive index of the solution compared to the pure solvent. Their sensitivity therefore depends on how much the refractive indices of the pure solvent and the dissolved substance differ. Due to this generally rather small difference, the detector sensitivity is rather low compared to UV absorption detectors, for example.
Refractive index detectors can be used for substances that do not absorb in the UV range and cannot be detected with conductivity detectors, for example when analyzing sugars. In the gel permeation chromatography of polymers, RI detectors are often used because of their simple design. This is because the refractive index of polymers is constant for different molar masses, and detection is therefore quantitative.
Charged aerosol detector CAD Detector
The Charged Aerosol Detector (CAD) is a detector used to measure the amount of chemicals in a sample by generating charged aerosol particles that are detected with an electrometer. The CAD can be used to measure all non-volatile and many semi-volatile analytes, including, but not limited to, antibiotics, excipients, ions, lipids, natural products, biofuels, sugars, and surfactants. The CAD, like other aerosol detectors (e.g., evaporative light scattering detectors (ELSD)), belongs to the category of destructive general-purpose detectors.
Evaporative Light Scattering Detector - ELSD Detector
An evaporative light scattering detector is used to detect compounds that can only be detected with difficulty or weakly in the UV/VIS range. The signal intensity of the detector is largely determined by the molar mass of the substance to be detected, and not by its chemical composition. Functional groups also have little influence. Therefore, substances with similar molar masses also have similar signal strengths. The detector is highly suitable for gradient systems, as UV or refractive index properties have no influence on the baseline. Another advantage is that the substance to be detected does not have to have chromophores, as is necessary for detection using UV or fluorescence detectors, for example.
Selective detectors, on the other hand, only detect certain components with special properties (e.g. UV-active substances, fluorescent compounds, ionic samples). Typical examples of selective detectors include:
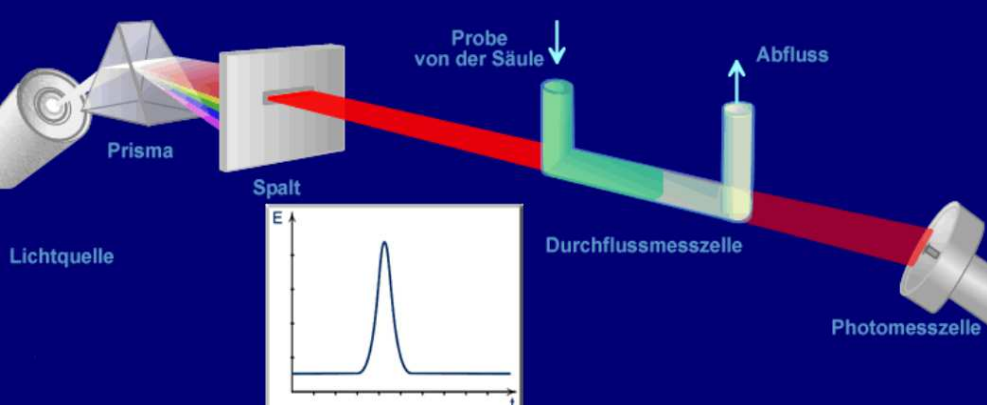
UV/VIS Detector UV/VIS Detector - UV radiation - visual light
The UV/VIS detector only detects analytes that absorb sufficient UV radiation or visible light (VIS). Many substances absorb at the short wavelengths around 200 nm, but so does the eluent in most cases. The analyte’s absorption maximum should be at a longer wavelength than the eluent absorption. The UV/VIS detector is used, for example, for (polycyclic) aromatics, conjugated double bond systems, double and multiple bonds, carbonyl groups C=O
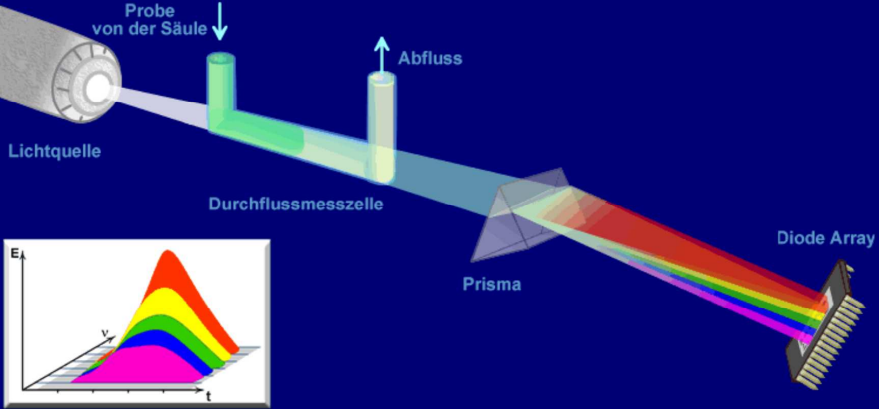
Dioden array Detektor DAD Detector
The DAD detector is a UV/VIS detector that covers the entire range from 190-800 nm. This offers greater flexibility compared to the UV/VIS detector, as the wavelength does not have to be known in advance.
difference between HPLC UV/VIS and HPLC DAD
The UV/VIS detector detects a specific wavelength (either at a fixed wavelength or a variable one) monochromatically. In contrast, the DAD detector is excited by a polychromatic light source. The entire spectrum is detected on the diode array at any given time. The flexibility of the DAD detector means that it is used more frequently in method development, for example.
Fluoreszenz-Detector FLD Detector - fluorescent light detector
FLD is a very sensitive detector that is 100-1000 times more sensitive than a UV/VIS detector. This detector is dependent on the chemical nature of the analytes. It can only be used with fluorescent analytes such as polycyclic aromatics. Therefore, it cannot be used universally.
electrochemical detector ECD Detector - electro chemical detector
Electrochemical detectors include amperometric and coulometric detectors. Electrochemical detection is based on the measurement of the current flow between two electrodes during the oxidation or reduction of analyte molecules. For certain substance classes, electrochemical detectors are much more sensitive than UV/VIS detectors and for some substances (e.g. the eurotransmitters from the catecholamine group) they are by far the most sensitive detection method.
Conductivity Detector HPLC CD - is CD correct? CD does not stand for Circular Dichroism
The detection of substances based on differences in the conductivity of the mixture “analyte + eluent” is particularly important in ion analysis. The main difficulty lies in the high basic conductivity of the buffer solutions used as the mobile phase. Therefore, suppressor columns are installed in front of the detector, in which the charge of the buffer salts is neutralized.
Specialized detectors are so selective that they not only detect a sample substance, but are also able to determine certain structures or elements with a high degree of reliability. These specialized selective detectors include:
mass spectrometric detectors such as ESI Detector - Electricsprayionization
ESI is particularly suitable for polar molecules, especially those with functional groups that can be easily protonated or deprotonated, which applies to many molecules, including biopolymers. In contrast, nonpolar molecules, such as unfunctionalized polycyclic aromatics, cannot be analyzed by ESI without prior derivatization.
mass spectrometric detectors such as APCI Detector - atmospheric pressure chemical ionization
The APCI method, which is related to ESI, is also suitable for nonpolar molecules. Here, too, the eluate is finely sprayed using a capillary and a nebulizer gas. In contrast to ESI, however, no high voltage is applied to the capillary; instead, the capillary is heated strongly.
Fourier transform infrared spectrometric detector FTIR Detector
In principle, an IR detector is similar to a UV/VIS detector, whereby
infrared radiation is sent through a cuvette and the IR absorption spectrum
of the “eluent + analyte” solution is recorded. Since the solvent also has IR absorption bands, it must be carefully selected so as not to mask the analyte bands.
Due to these limitations, an IR detector can often
be used to its full extent. The detection strength of IR detectors is rather low
and comparable to that of refractive index detectors.
nuclear magnetic resonance Detector NMR Detector
Due to the possibilities for structure elucidation, nuclear magnetic resonance
(NMR) is, in principle, an excellent detection method. However, NMR is not very sensitive, which makes technically complex
“stopped-flow” equipment necessary, since an NMR measurement can take minutes. Another requirement is the use of deuterated
solvents. Since NMR is also an expensive method, it is rarely
directly coupled with LC in practice.
Method development HPLC
The goal of HPLC and UPLC method development is to separate a given number of analytes in the shortest possible time and at the best possible resolution. At the same time, the methods should be reproducible and robust so that identical results can be obtained at different times and in different places.
A chromatographic method to be developed always consists of the interdependent three components:
- Sample
- Stationary phase (separation column
- Mobile phase (eluent)
These three components of the chromatographic system must be optimally combined with each other in order to ultimately obtain the best possible analytical method for a given separation problem.
Based on the separation problem to be solved and the properties of the sample to be analyzed, the following principal points belong to a method development:
- Selection of separation technique (RP, NP, HILIC, SEC, IEX, etc.)
- Selection of column dimensions and LC (HPLC or UPLC)
- Selecting the stationary phase (silica gel, RP18, chiral column, GPC column, etc.)
- Selecting the mobile phase and adjusting its pH (if necessary)
- Running isocratic or gradient runs
- Further optimizing the parameters, e.g. by varying
- the temperature
- of the buffer or ion pair reagent
- the flow rate
- of the gradient
- …
What is HPLC used for?
Liquid chromatography (LC) is used in many areas, both analytically for the identification and quantification of substances or the determination of molecular weight distributions, and preparatively for the separation and purification of mixtures of substances.
Due to the wide range of stationary phases, separation mechanisms and detectors, the scope of application extends from small inorganic or organic ions, neutral organic molecules and organometallic complexes to large biomolecules (e.g. proteins).

